Address
9841 Washingtonian Blvd, Suite 200 Gaithersburg, MD 20878, USA
Situation
Regulatory agencies increasingly recognize that traditional animal models often fail to predict human responses in drug safety assessment. To improve human relevance, the FDA is investing heavily in:
- Organ-on-chip platforms
- 3D tissue constructs
- Microfluidic lung and kidney systems
- MPS-derived biomarkers
These models are emerging as New Approach Methodologies (NAMs) and potential sources of mechanistic real-world evidence, particularly useful where clinical data is limited or human trials are risky.
Cognizance scientific teams played a major role in generating reproducible MPS data, validating system performance, and providing interpretive frameworks for regulatory use.
Challenge
Despite their promise, Microphysiological Systems face several adoption barriers:
1. Lack of standardized testing workflows – Different labs operate MPS devices differently, producing variable outputs.
2. Difficulty comparing MPS outputs across platforms – No single “reference standard” exists for many endpoints.
3. Limited understanding of translational validity – Regulators need to know if an MPS model predicts human physiology reliably.
4. Need for scalable readouts – To be regulatory-useful, assays must work with reproducible biomarkers, analytics, or AI-enabled interpretation.
5. Data complexity – MPS outputs are multidimensional (electrical, biochemical, morphological) and require sophisticated analytics.
These gaps limited regulatory acceptance – until systematic validation and benchmarking work began to address them.
Solution
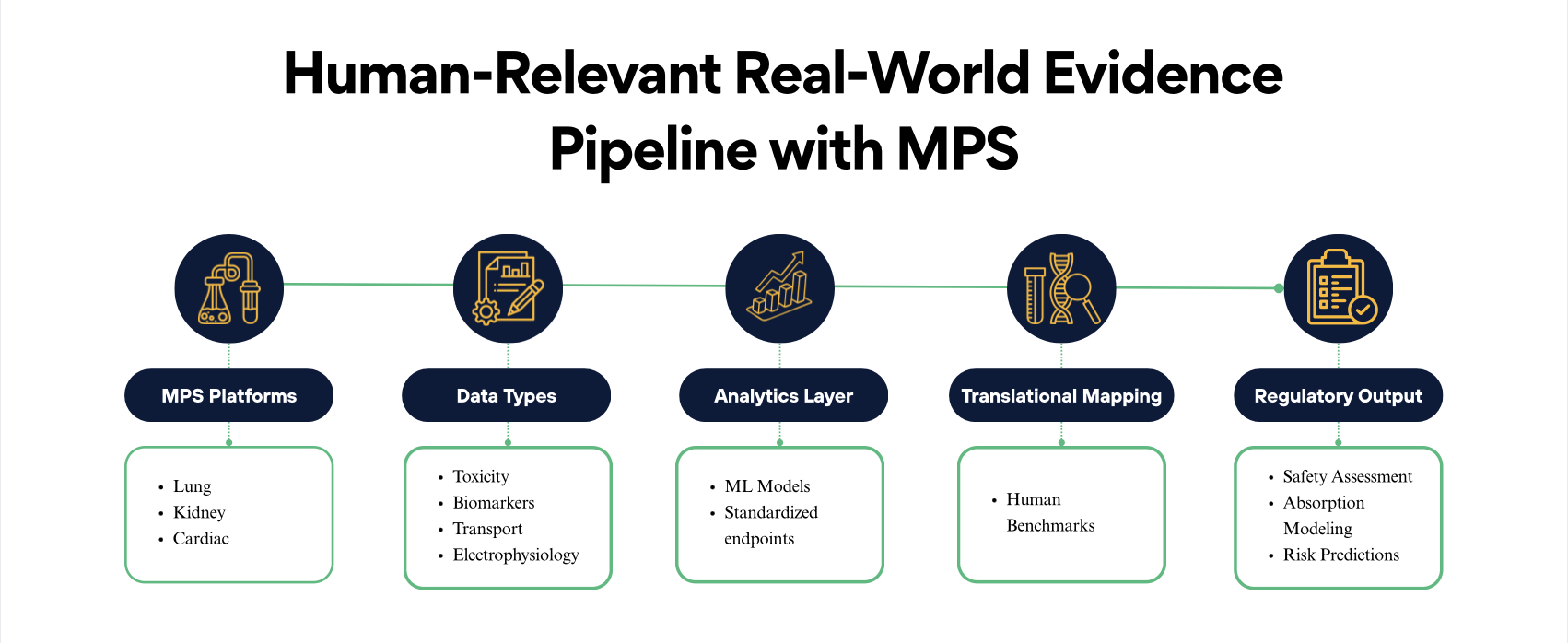
Cognizance scientific teams helped FDA advance the regulatory use of MPS by developing and validating several high-fidelity organ systems and analytic frameworks.
1. Lung Microphysiological Systems for Drug Absorption & Safety
- Small airway MPS to study permeability of inhaled therapeutics
- 3D reconstructed airway tissues for toxicant and inflammation studies
- Quantitative absorption and permeability models
- Cross-laboratory reproducibility metrics
Regulatory impact: Supports OINDP (Orally Inhaled and Nasal Drug Products) evaluations for absorption, safety, and local toxicity.
2. Kidney MPS for Toxicity Biomarker Validation
- Proximal tubule models to assess nephrotoxicity
- Side-by-side comparisons of 2D vs 3D models
- Discovery and validation of translational safety biomarkers
- Standardizing injury endpoints across platforms
Regulatory impact: Provides stronger human-relevant data for drug-induced kidney injury assessments.
3. Cardiac Safety NAMs & NAM-MPS Integration
- Cardiac microtissues for predicting pro-arrhythmic risk
- NAM-based approaches for drug–drug interaction safety modeling
- Integration of electrophysiological and biochemical readouts
Regulatory impact: Supports CDER’s movement toward in vitro cardiac safety platforms beyond the hERG assay.
4. Machine Learning Approaches to Standardize MPS Interpretation
Cognizance’s scientific teams contributed to best practices for:
- Feature selection
- Predictive modeling workflows
- Benchmarking guidelines
- Standardizing ML approaches across MPS platforms
Regulatory impact: Establishes transparent, reproducible data pipelines compatible with regulatory workflows.
5. Frameworks for Regulatory Adoption
Contributions include:
- Workshops and consensus papers on best practices
- Guidance on performance qualification
- Recommendations for cross-platform comparisons
Regulatory impact: Aligns MPS development with FDA expectations for reproducibility and translational performance.
Results
Cognizance’s work helped advance MPS as credible sources of real-world evidence for regulatory science.
✔ Demonstrated human-relevant mechanistic insights – Lung and kidney MPS accurately reproduced transport, toxicity, and permeability phenomena observed in humans.
✔ Validated translational biomarkers – Kidney and lung injury biomarkers showed predictive alignment with known human responses.
✔ Increased reproducibility and standardization – Harmonized workflows and ML frameworks reduced variability across labs.
✔ Enabled FDA reviewers to use MPS data – Submissions now include MPS-derived data for absorption, toxicity, and mechanistic interpretation.
✔ Enhanced drug safety predictions – NAM-based MPS systems supported early hazard identification, reducing late-stage failures and unnecessary animal use.
These contributions push MPS forward as part of the next generation of regulatory science tools.
